Introduction: Targeted agents, in particular BCL2-inhibitors (BCL2i) and BTK-inhibitors (BTKi), have profoundly improved the outcome of patients (pts) with chronic lymphocytic leukemia (CLL) and are considered standard treatment. However, the transition from treatments evaluated within clinical trials into routine clinical practice can be complex. We here present real-world data from the German CLL Study Group (GCLLSG) registry and report outcomes and treatment sequences of pts treated with either BCL2i (venetoclax) or BTKi (acalabrutinib, ibrutinib, spebrutinib, tirabrutinib, or zanubrutinib) as first administered targeted agent.
Methods: Pts from multiple German sites with confirmed diagnosis of CLL and with at least one documented first-line CLL treatment between July 1 st 2014 and January 30 th 2023 were included in this analysis. Pts participating in an active clinical trial were excluded. Treatments were categorized according to the backbone of therapy and pts were allocated to either the venetoclax (Ven) or the BTKi cohort depending on their first treatment with one of these substances. Time to next treatment (TTNT), event-free survival (EFS) and overall survival (OS) were analyzed using Kaplan-Meier methods.
Results: A total of 274 pts were treated with a Ven-based regimen while 915 pts were treated with a BTKi as first therapy. Median observation time from the first documented treatment (irrespective if given as first-line or in a later therapy line) was 26 months (range 0 - 296) in the Ven cohort and 78 months (range 0-266) in the BTKi cohort.
For the Ven cohort, median age at the time of first Ven treatment was 71 years (range 32 - 87) with 172 pts (62.8%) being male. Median CIRS score was 2 (range 0-13) and 12 pts (7.5%) had an ECOG performance status > 1. Among 57 pts with known molecular genetics, 32 pts (56.1%) had an unmutated IGHV status and 9 out of 51 evaluable pts (17.6%) had a deletion and/or mutation TP53. Applying the CLL-IPI, 11 out of 58 evaluable pts (19.0%) were in the very high-risk group.
In the BTKi cohort, median age at time of first BTKi treatment was 72 (range 32 - 92), with 628 pts (68.6%) being male. Median CIRS score was 3 (range 0-17) and 45 pts (9.5%) had an ECOG performance status > 1. Among 162 pts with available data, 108 pts (66.7%) had an unmutated IGHV status and 59 out of 130 evaluable pts (45.4%) had a deletion and/or mutation TP53. Using the CLL-IPI, 46 out of 145 evaluable pts (31.7%) were in the very high-risk group.
A total of 152 of 274 pts (55.5%) in the Ven cohort received Ven as first-line treatment (median number of prior treatments: 0, range 0-9) and 352 of 915 pts (38.5%) in the BTKi cohort received a BTKi as first-line treatment (median number of prior treatments: 1, range 0-12).
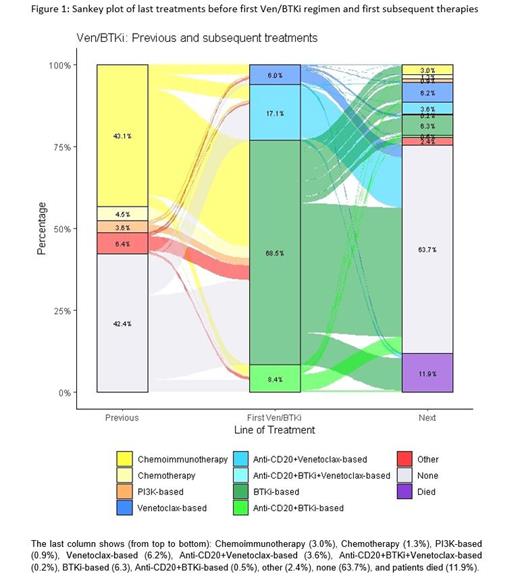
Among all 222 administered treatments prior to first Ven and 1090 treatments administered prior to first BTKi, chemoimmunotherapy (70.3% and 68.3%, respectively) and chemotherapy (10.4% and 17.8%, respectively) were the most frequent.
For subsequent therapy following the first documented Ven or BTKi treatment, most pts were treated with targeted agent regimens. In the Ven cohort, 37 subsequent treatments were documented in 26 pts, of which 10 (27.0%) were a Ven-containing regimen and 13 (35.1%) a BTKi-containing regimen. Within the BTKi cohort, 393 subsequent treatments were administered in 264 pts, of which 146 (37.2%) included Ven and 106 (27.0%) a BTKi Treatment sequences were visualized with a sankey-diagram ( Figure 1).
Median EFS from the start of the first documented Ven treatment was 31.8 months, with an estimated 2-year EFS rate of 66.6%. Median TTNT was 63.7 months and median OS was 96.5 months with an estimated 2-year OS rate of 89.5%. When Ven was given as first-line treatment, the estimated 2-year OS rate was 93.6% versus 86.5% when given in a later therapy line for the first time.
For the BTKi cohort, median EFS from start of first documented BTKi treatment was 23.2 months, with an estimated 2-year EFS rate of 48.6%. Median TTNT was 68.4 months and median OS was 85.9 months with an estimated 2-year OS rate of 84.9%. When BTKi were given as first-line treatment, the estimated 2-year OS rate was 92.3% versus 81.3% when given in a later therapy line for the first time.
Conclusion: EFS, TTNT and OS were comparable following Ven versus BTKi-based therapy in pts documented in the GCLLSG registry between 2014 and 2023.
OffLabel Disclosure:
Kutsch:Gilead: Honoraria, Research Funding; AstraZeneca: Honoraria; Janssen: Other: Travel support; Celgene: Other: Travel support; AbbVie: Honoraria, Other: Travel support; BMS: Honoraria; Beigene: Other: Travel support. Illmer:AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Al-Sawaf:Ascentage: Membership on an entity's Board of Directors or advisory committees; Adaptive: Speakers Bureau; BeiGene: Research Funding, Speakers Bureau; Eli Lilly: Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Langerbeins:AstraZeneca: Honoraria, Other: travel support; Beigene: Honoraria, Other: travel support; Janssen: Honoraria, Other: travel support, Research Funding; Abbvie: Honoraria, Other: travel support. Cramer:BMS: Honoraria; Gilead: Research Funding; Novartis: Research Funding; BeiGene: Consultancy, Other: travel support, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: travel support, Research Funding; Janssen: Honoraria, Research Funding; Acerta: Research Funding; Abbvie: Consultancy, Honoraria, Other: travel support, Research Funding; Roche: Honoraria, Other: travel support, Research Funding. Eichhorst:Abbvie: Consultancy, Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; BeiGene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gilead: Consultancy, Research Funding; Janssen: Consultancy, Research Funding, Speakers Bureau; Lilly: Consultancy, Speakers Bureau; MSD: Consultancy, Honoraria, Speakers Bureau; F. Hoffmann-La Roche Ltd: Honoraria, Research Funding, Speakers Bureau. Hallek:Abbvie: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding. Fischer:Roche: Honoraria, Other: Travel Support; AstraZeneca: Consultancy; Abbvie: Honoraria, Other: TRavel support. Fink:AbbVie: Other: Travel Support; Celgene: Research Funding; Astra Zeneca: Honoraria, Research Funding.
spebrutinib, tirabrutinib not commercially available for CLL